At gas phase concentrations greater than 30% volume in air/ at partial pressures above 10 k Pa, ClO2 may explosively decompose into chlorine and oxygen. The decomposition can be initiated by, for example, light, hot spots, chemical reaction, or pressure shock. Thus, chlorine dioxide gas is never handled in concentrated form, but is almost always handled as a dissolved gas in water in a concentration range of 0.5 to 10 grams per litre. Its solubility increases at lower temperatures: it is thus common to use chilled water (5 °C or 41 °F) when storing at concentrations above 3 grams per liter.
In many countries, such as the USA, chlorine dioxide gas may not be transported at any concentration and is almost always produced at the application site using a chlorine dioxide generator. In some countries, chlorine dioxide solution below 3 grams per liter in concentration may be transported by land, but are relatively unstable and deteriorate quickly. The use of chlorine dioxide generators is steadily falling out of fashion in industry, as these systems generally require the use of strong acids to work and can take several hours to reach their full yield with poor efficiency. The requirement to store the hazardous gas in a pressurized chamber poses a risk some sites prefer not to take.
Can chlorine dioxide be dissolved in water?
One of the most important qualities of chlorine dioxide is its high water solubility, especially in cold water. Chlorine dioxide does not hydrolyses when it enters water; it remains a dissolved gas in solution. Chlorine dioxide is approximately 10 times more soluble in water than chlorine. Chlorine dioxide can be removed by aeration or carbon dioxide.
How can chlorine dioxide be stored?
The best way to store chlorine dioxide is as a liquid at 4 ºC. At this state it is fairly stable. Chlorine dioxide cannot be stored for too long, because it slowly dissociates into chlorine and oxygen. It is rarely stored as a gas, because it is explosive under pressure. When concentrations are higher than 10% chlorine dioxide in air, there is an explosion hazard. In a watery solution, chlorine dioxide remain stable and soluble. Watery solutions containing approximately 1% ClO2 (10 g/L) can safely be stored, under the condition that they are protected from light and heat interference. Chlorine dioxide is rarely transported, because of its explosiveness and instability. It is usually manufactured on site.
Does the pH value influence chlorine dioxide efficiency?
Contrary to chlorine, chlorine dioxide is effective at a pH of between 5 and 10. The efficiency increases at high pH values, while the active forms of chlorine are greatly influenced by pH. Under normal circumstances chlorine dioxide does not hydrolyze. This is why the oxidation potential is high and the disinfection capacity is not influenced by pH. Both temperature and alkalinity of the water do not influence the efficiency. At the concentrations required for disinfection, chlorine dioxide is not corrosive. Chlorine dioxide is more water-soluble than chlorine. In the last few years better and safer methods for chlorine dioxide production have been developed.
Chlorine dioxide as an oxidizer
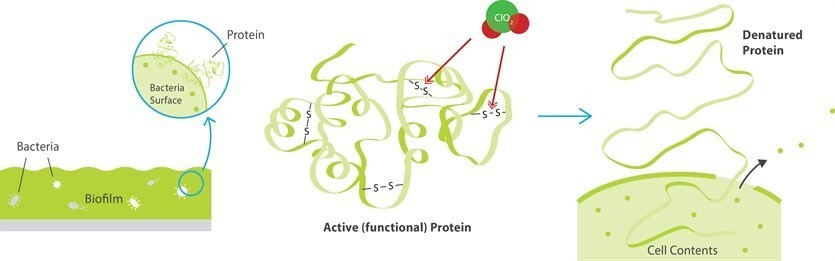
As an oxidizer chlorine dioxide is very selective. It has this ability due to unique one-electron exchange mechanisms. Chlorine dioxide attacks the electron-rich centers of organic molecules. One electron is transferred and chlorine dioxide is reduced to chlorite (ClO2– ).
Figure 2: chlorine dioxide is more selective as an oxidizer than chlorine. While dosing the same concentrations, the residual concentration of chlorine dioxide is much higher with heavy pollution than the residual concentration of chlorine.
By comparing the oxidation strength and oxidation capacity of different disinfectants, one can conclude that chlorine dioxide is effective at low concentrations. Chlorine dioxide is not as reactive as ozone or chlorine and it only reacts with sulphuric substances, amines and some other reactive organic substances. In comparison to chlorine and ozone, less chlorine dioxide is required to obtain an active residual disinfectant. It can also be used when a large amount of organic matter is present.
The oxidation strength describes how strongly an oxidizer reacts with an oxidizable substance. Ozone has the highest oxidation strength and reacts with every substance that can be oxidized. Chlorine dioxide is weak, it has a lower potential than hypochlorous acid or hypobromous acid.
The oxidation capacity shows how many electrons are transferred at an oxidation or reduction reaction. The chlorine atom in chlorine dioxide has an oxidation number of +4. For this reason chlorine dioxide accepts 5 electrons when it is reduced to chloride. When we look at the molecular weight, chlorine dioxide contains 263 % ‘available chlorine’; this is more than 2,5 times the oxidation capacity of chlorine.
The following comparisons show what happens when chlorine dioxide reacts. First, chlorine dioxide takes up an electron and reduces to chlorite:
ClO2 + e- ® ClO2–
The chlorite ion is oxidized and becomes a chloride ion:
ClO2– + 4H+ + 4e- ® Cl– + 2H2O
These comparisons suggest that chlorine dioxide is reduced to chloride, and that during this reaction it accepts 5 electrons. The chlorine atom remains, until stable chloride is formed. This explains why no chlorinated substances are formed. When chlorine reacts it does not only accept electrons; it also takes part in addition and substitution reactions. During these reactions, one or more chlorine atoms are added to the foreign substance.
Features Of Chlorine Dioxide
- Effective and full elimination of all known in water common microorganisms, i.e. bacteria (incl. Gardia, legionella), viruses (incl. hepatitis, anthrax), protozoan (incl. Crypto and Giardia), yeast, fungi, algae and cysts.
- Stronger, efficacious and faster disinfection capacity.
- 260% more disinfection power compared to chlorine.
- 10 times more oxidizing power compared to chlorine.
- Long lasting residual disinfection capacity throughout water systems (up to 72 hours, chlorine only 2-6 hours)
- Highly soluble in water.
- Steady bactericidal action within a broad pH bandwith (pH 4-10).
- Very short contact time.(2-10 minutes).
- No build-up of resistance by microorganisms.
- Higher efficiency in the removal of iron and manganese compounds.
- Destroys algae related taste and odour compounds.
- Extensive kinetic half time.
- Less sewage load by strong decrease in building of organic halogen combinations, provides THM control.
- High operational safety.
- No adverse taste and odour effects in the disinfected water.
- Bounded chlorine building is strongly suppressed.
- Does not react with water to form hypochlorous acid.
- ClO2 does not react with ammonia, ammonium or most organic compounds.
- Decreases, THM’s, HAA’s, MX and other organic compounds with toxic or carcinogenic properties.
- Removes biofilm very effectively from pipeline & various system parts.